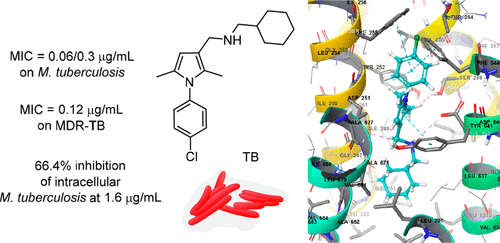
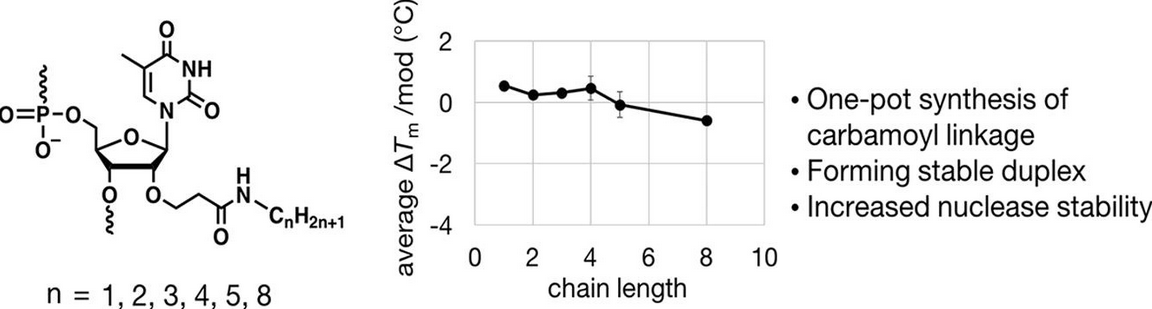
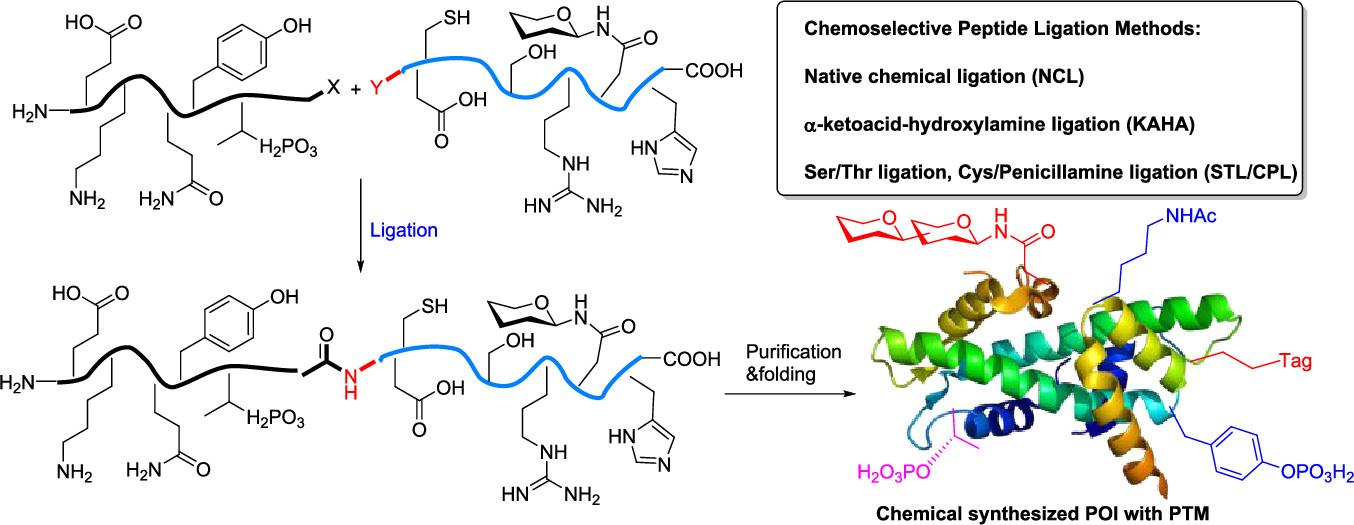
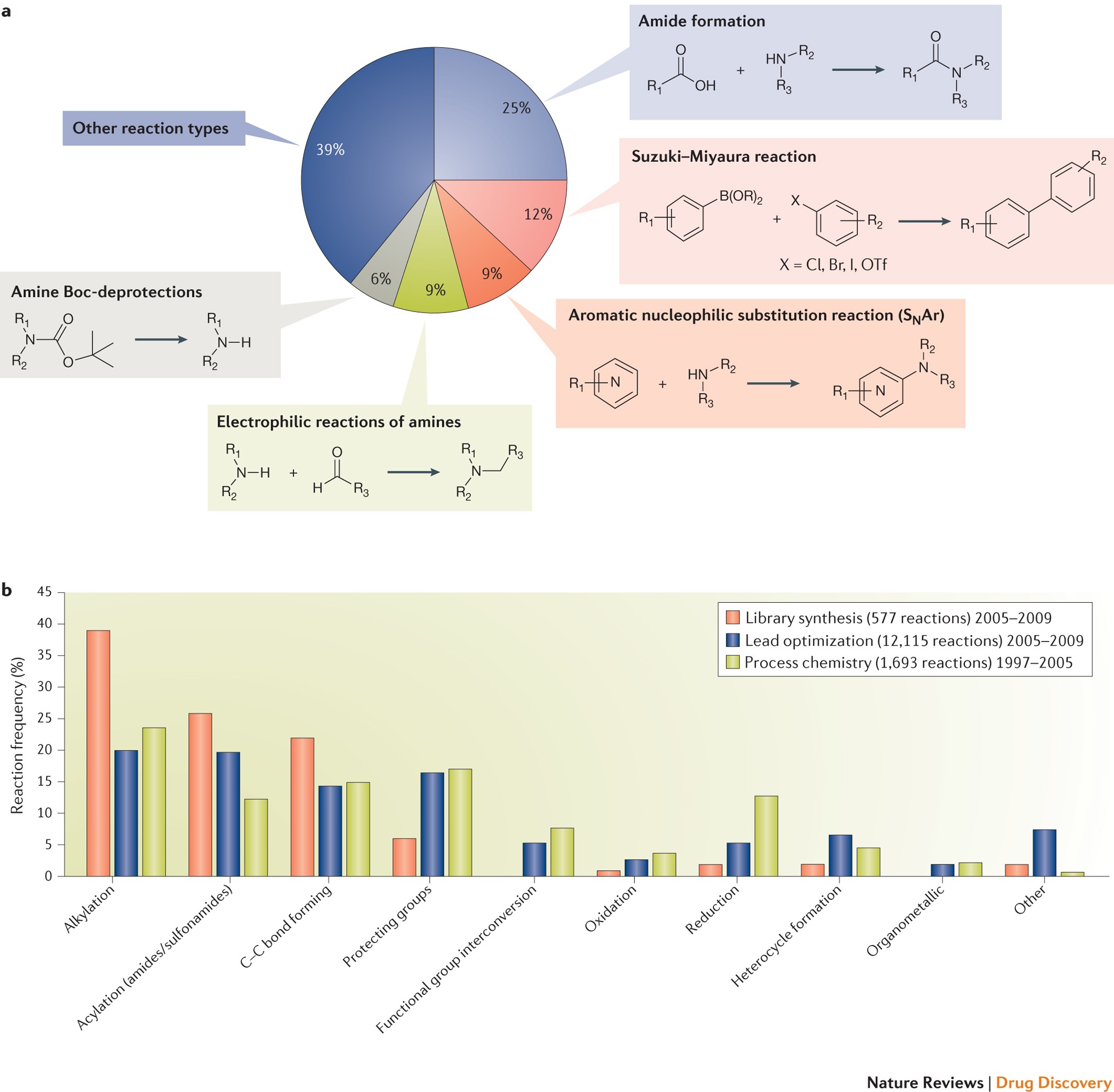
Direct-to-Biology Accelerates PROTAC Synthesis and the Evaluation of Linker Effects on Permeability and Degradation | ACS Medicinal Chemistry Letters
![PDF) Corrigendum to “Bone-Targeted Src Kinase Inhibitors: Novel Pyrrolo- and Pyrazolopyrimidine Analogues”[Bioorg. Med. Chem. Lett. 13 (2003) 3063] | Raji Sundaramoorthi - Academia.edu PDF) Corrigendum to “Bone-Targeted Src Kinase Inhibitors: Novel Pyrrolo- and Pyrazolopyrimidine Analogues”[Bioorg. Med. Chem. Lett. 13 (2003) 3063] | Raji Sundaramoorthi - Academia.edu](https://0.academia-photos.com/attachment_thumbnails/39993522/mini_magick20190222-26208-17ntcpo.png?1550829966)
PDF) Corrigendum to “Bone-Targeted Src Kinase Inhibitors: Novel Pyrrolo- and Pyrazolopyrimidine Analogues”[Bioorg. Med. Chem. Lett. 13 (2003) 3063] | Raji Sundaramoorthi - Academia.edu
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commerci

Our paper interrogating the NMR binding detection limits of fragments targeting the VHL-HIF1α PPI is now published in ACS Med. Chem. Lett. | Ciulli Laboratory

PDF) Synthesis of antitrypanosomal 1,2-dioxane derivatives based on a natural product scaffold | Ngoc Pham - Academia.edu
Discovery of novel 2-[2-(3-hydroxy-pyridin-2-yl)-thiazol-4-yl]-acetamide derivatives as HIF prolyl 4-hydroxylase inhibitors; SAR
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commerci

Synthesis, Biological Evaluation, and Molecular Docking of Arylpyridines as Antiproliferative Agent Targeting Tubulin | ACS Medicinal Chemistry Letters